Welcome to DU!
The truly grassroots left-of-center political community where regular people, not algorithms, drive the discussions and set the standards.
Join the community:
Create a free account
Support DU (and get rid of ads!):
Become a Star Member
Latest Breaking News
Editorials & Other Articles
General Discussion
The DU Lounge
All Forums
Issue Forums
Culture Forums
Alliance Forums
Region Forums
Support Forums
Help & Search
Did a genetic mutation lead to a woman being convicted of murdering her four children? [View all]
Here's an interesting article in Nature News Feature I came across today:
She was convicted of killing her four children. Could a gene mutation set her free?
Subtitle:
Kathleen Folbigg has spent 19 years in prison and was dubbed ‘Australia’s worst female serial killer’. Now, an inquiry into her case will look at clinical genetics in a whole new way.
Some excerpts:
Around lunchtime on a warm March day in 1999, Kathleen Folbigg went to check on her sleeping 18-month-old daughter and found her pale and unresponsive. Folbigg, alone in her house in Singleton, Australia, called an ambulance while she tried her best to resuscitate the child. “My baby’s not breathing,” she said, pleading for them to hurry.
“I’ve had three SIDS deaths already,” she explained, referring to sudden infant death syndrome — a largely unexplained phenomenon that typically affects infants in their first year, as they sleep.
Around 9 p.m. that night, pathologist Allan Cala conducted an autopsy on the baby, named Laura, at the New South Wales Institute of Forensic Medicine in Glebe, a suburb of Sydney. In his report, he noted no evidence of injury and no medications, drugs or alcohol in her system. He mentioned some inflammation of the heart, possibly caused by a virus, but surmised that it could be incidental. Instead, Cala opined on the improbability of four children in the same family dying from SIDS. “The possibility of multiple homicides in this family has not been excluded,” the report stated.
Four years later, in May 2003, a jury found Folbigg guilty of murdering three of her children — Patrick, Sarah and Laura — and of the manslaughter of her first son, Caleb. Because there were no physical signs of foul play in any of the deaths, the case had rested entirely on circumstantial evidence, including the unlikelihood of four unexplained deaths occurring in one household. Lightning doesn’t strike the same person four times, the prosecutor told the jury.
Folbigg was sentenced to 40 years in prison, and became known as Australia’s worst female serial killer. But in 2018, a group of scientists began gathering evidence that suggested another possibility for the deaths — that at least two of them were attributable to a genetic mutation that can affect heart function. A judicial inquiry in 2019 failed to reverse Folbigg’s conviction, but this month, the researchers will present a bolus of new evidence at a second inquiry, which could ultimately end in freedom for Folbigg after nearly 20 years behind bars. More than 90 scientists signed a March 2021 petition arguing for her release on the basis of that evidence.
The inquiry will have to grapple with how science weighs the evidence for genetic causes of disease, and how that fits with the legal system’s concept of reasonable doubt. But it will have help. Thomas Bathurst, the retired judge leading the inquiry who will decide Folbigg’s fate, has granted permission for the Australian Academy of Science in Canberra to act as an independent scientific adviser. The academy will recommend experts to give evidence, and will look at questions asked of those experts to ensure their scientific accuracy.
This will probably present the science more accurately than at the original trial, says Jason Chin, a legal academic at the University of Sydney who studies the way science is used in courts. And this case could have implications for how Australian legal proceedings consider scientific evidence in other cases, says Chin.
Sudden suspicion
Folbigg’s four children died over a period of ten years. Caleb was just 19 days old in 1989 at the time of his death. Patrick and Sarah were 8 and 10 months old, respectively. Soon after Laura’s death, Folbigg was placed under suspicion and eventually stood trial in a case that became a dramatic public spectacle. At the time, multiple SIDS deaths in a single family were viewed with suspicion, particularly against mothers.
That suspicion traces at least in part to Roy Meadow, a British paediatrician who studied child abuse. In 1997, he popularized the idea that “one sudden infant death is a tragedy, two is suspicious and three is murder, unless proven otherwise”. At Folbigg’s trial, this line of thinking clearly influenced some experts’ testimony...
“I’ve had three SIDS deaths already,” she explained, referring to sudden infant death syndrome — a largely unexplained phenomenon that typically affects infants in their first year, as they sleep.
Around 9 p.m. that night, pathologist Allan Cala conducted an autopsy on the baby, named Laura, at the New South Wales Institute of Forensic Medicine in Glebe, a suburb of Sydney. In his report, he noted no evidence of injury and no medications, drugs or alcohol in her system. He mentioned some inflammation of the heart, possibly caused by a virus, but surmised that it could be incidental. Instead, Cala opined on the improbability of four children in the same family dying from SIDS. “The possibility of multiple homicides in this family has not been excluded,” the report stated.
Four years later, in May 2003, a jury found Folbigg guilty of murdering three of her children — Patrick, Sarah and Laura — and of the manslaughter of her first son, Caleb. Because there were no physical signs of foul play in any of the deaths, the case had rested entirely on circumstantial evidence, including the unlikelihood of four unexplained deaths occurring in one household. Lightning doesn’t strike the same person four times, the prosecutor told the jury.
Folbigg was sentenced to 40 years in prison, and became known as Australia’s worst female serial killer. But in 2018, a group of scientists began gathering evidence that suggested another possibility for the deaths — that at least two of them were attributable to a genetic mutation that can affect heart function. A judicial inquiry in 2019 failed to reverse Folbigg’s conviction, but this month, the researchers will present a bolus of new evidence at a second inquiry, which could ultimately end in freedom for Folbigg after nearly 20 years behind bars. More than 90 scientists signed a March 2021 petition arguing for her release on the basis of that evidence.
The inquiry will have to grapple with how science weighs the evidence for genetic causes of disease, and how that fits with the legal system’s concept of reasonable doubt. But it will have help. Thomas Bathurst, the retired judge leading the inquiry who will decide Folbigg’s fate, has granted permission for the Australian Academy of Science in Canberra to act as an independent scientific adviser. The academy will recommend experts to give evidence, and will look at questions asked of those experts to ensure their scientific accuracy.
This will probably present the science more accurately than at the original trial, says Jason Chin, a legal academic at the University of Sydney who studies the way science is used in courts. And this case could have implications for how Australian legal proceedings consider scientific evidence in other cases, says Chin.
Sudden suspicion
Folbigg’s four children died over a period of ten years. Caleb was just 19 days old in 1989 at the time of his death. Patrick and Sarah were 8 and 10 months old, respectively. Soon after Laura’s death, Folbigg was placed under suspicion and eventually stood trial in a case that became a dramatic public spectacle. At the time, multiple SIDS deaths in a single family were viewed with suspicion, particularly against mothers.
That suspicion traces at least in part to Roy Meadow, a British paediatrician who studied child abuse. In 1997, he popularized the idea that “one sudden infant death is a tragedy, two is suspicious and three is murder, unless proven otherwise”. At Folbigg’s trial, this line of thinking clearly influenced some experts’ testimony...
Meadow's claim was already under suspicion:
...In fact, by the time the trial started, scientists had been expressing concern about Meadow’s idea for a number of years, and particularly its use in legal cases. Sally Clark, a mother jailed under similar circumstances in the United Kingdom, had successfully challenged her conviction partly on the basis that Meadow’s arguments were statistically unsound and made unsupported assumptions about the rate of SIDS. The idea was largely discredited by 2003, and Meadow was eventually struck from the UK medical register in 2005 because of misleading testimony he had offered during Clark’s trial...
After Ms Folbigg has spent 19 years in prison, a possible genetic cause of the children's deaths is now being considered:
Genetics enters the picture
As part of their preparations for the inquiry, Folbigg’s lawyers approached Carola Vinuesa, a geneticist at the Australian National University (ANU) in Canberra at the time, to sequence and analyse Folbigg’s DNA. The idea was to see whether she carried any mutations that, if inherited by the children, might offer an alternative explanation for how they died. Vinuesa agreed to help. Her colleague, Todor Arsov, a geneticist who lived in Sydney, travelled to the nearby Silverwater Women’s Correctional Centre, where Folbigg was being held, to collect a sample from her.
That December, Arsov joined Vinuesa in her kitchen to scroll through sequence data looking for variants linked to sudden death. Within 20 minutes, they both came across something interesting: a variant in a gene called calmodulin 2 (CALM2).
Humans have three calmodulin genes, encoding identical proteins that bind to calcium and control its concentration in cells, which helps to regulate the heart’s contractions, among other things. Mutations in these genes are extremely rare, but people who have them often have serious cardiac conditions; sudden deaths have been reported. Vinuesa thought the find was worth further investigation. She suggested to Folbigg’s lawyers that they attempt to sequence DNA from the children and their father...
As part of their preparations for the inquiry, Folbigg’s lawyers approached Carola Vinuesa, a geneticist at the Australian National University (ANU) in Canberra at the time, to sequence and analyse Folbigg’s DNA. The idea was to see whether she carried any mutations that, if inherited by the children, might offer an alternative explanation for how they died. Vinuesa agreed to help. Her colleague, Todor Arsov, a geneticist who lived in Sydney, travelled to the nearby Silverwater Women’s Correctional Centre, where Folbigg was being held, to collect a sample from her.
That December, Arsov joined Vinuesa in her kitchen to scroll through sequence data looking for variants linked to sudden death. Within 20 minutes, they both came across something interesting: a variant in a gene called calmodulin 2 (CALM2).
Humans have three calmodulin genes, encoding identical proteins that bind to calcium and control its concentration in cells, which helps to regulate the heart’s contractions, among other things. Mutations in these genes are extremely rare, but people who have them often have serious cardiac conditions; sudden deaths have been reported. Vinuesa thought the find was worth further investigation. She suggested to Folbigg’s lawyers that they attempt to sequence DNA from the children and their father...
The children's tissues have been sequenced, but not that of the children's father:
...Scientists had managed to obtain DNA from the four children. A hospital in Sydney had Sarah’s frozen fibroblast cells from her autopsy in 1993, and the coroner’s court in Glebe had frozen liver tissue from Patrick’s 1991 autopsy. A laboratory in Melbourne sequenced a whole genome from one child’s heel-prick samples, which are routinely collected at birth, and retrieved just the protein-coding portion of the genome from another’s heel-prick card. Craig Folbigg declined to provide DNA...
Apparently the calmodulin gene is highly conserved, most mammals have an identical sequence to that of humans. This implies that mutations in the gene cannot be tolerated, that the expressed protein is not functional with modifications, and thus the gene does not evolve as other genes do.
Ms. Folbigg and her deceased children have a variant called G114R, where the 114th amino acid residue glycine (G) is replaced with arginine (R).
One of those researchers is Michael Toft Overgaard, a protein scientist at Aalborg University in Denmark, who was part of the team that discovered the first mutation in a calmodulin gene in 2012.
After the first inquiry, Vinuesa e-mailed Overgaard and asked whether he could perform a functional assay to determine the cellular effects of the Folbigg variant. Overgaard wasn’t familiar with the case, but was drawn to the idea of seeing “another piece in the puzzle to try and figure out how calmodulin works”, he says.
Overgaard asked postdocs Helene Halkjær Jensen and Malene Brohus to do the lab work. Everything about the project was secretive: even other researchers in their lab didn’t know about it. “We had a folder on our computer called CSI,” says Jensen, a reference to the popular US television drama about crime-scene investigators.
Jensen and Brohus spent weeks painstakingly making calmodulin proteins with the Folbigg mutation, known as G114R, in which the amino acid glycine (G) at the 114th position of the protein is replaced with an arginine (R). For comparison, they created proteins with two other calmodulin variants known to cause severe arrhythmias, G114W and N98S (see ‘Known mutations in calmodulin’).
After the first inquiry, Vinuesa e-mailed Overgaard and asked whether he could perform a functional assay to determine the cellular effects of the Folbigg variant. Overgaard wasn’t familiar with the case, but was drawn to the idea of seeing “another piece in the puzzle to try and figure out how calmodulin works”, he says.
Overgaard asked postdocs Helene Halkjær Jensen and Malene Brohus to do the lab work. Everything about the project was secretive: even other researchers in their lab didn’t know about it. “We had a folder on our computer called CSI,” says Jensen, a reference to the popular US television drama about crime-scene investigators.
Jensen and Brohus spent weeks painstakingly making calmodulin proteins with the Folbigg mutation, known as G114R, in which the amino acid glycine (G) at the 114th position of the protein is replaced with an arginine (R). For comparison, they created proteins with two other calmodulin variants known to cause severe arrhythmias, G114W and N98S (see ‘Known mutations in calmodulin’).
It is known that certain mutations in calmodulin lead to heart diseases, notably arrhythmias.
A figure from the article:
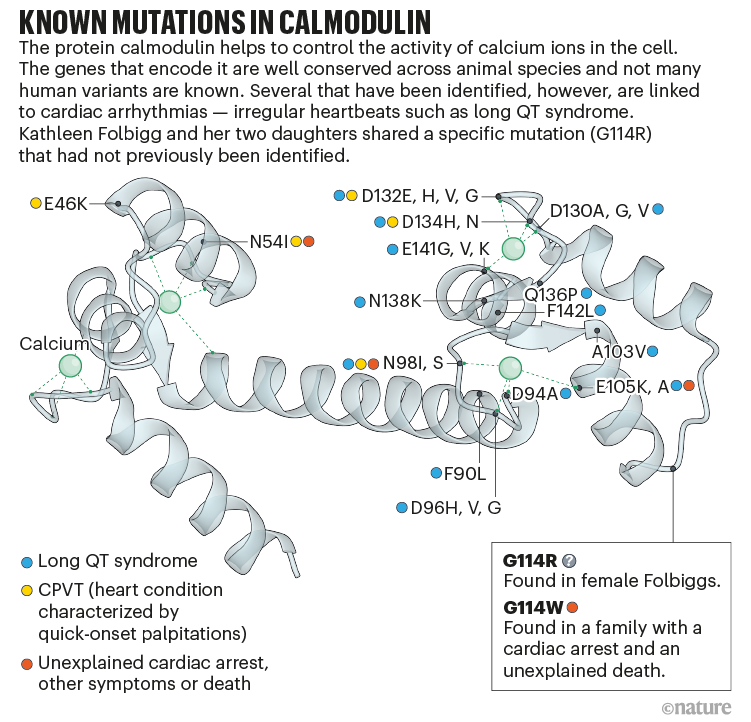
One of the first things they discovered was that the G114R variant cannot latch onto calcium effectively. The team thought this was important, because the effect was similar to that seen with the other two deadly variants. Further experiments revealed that G114R impairs how calmodulin attaches to two crucial channels that control the movement of calcium into the cell. Jensen says the results were compelling, but the team knew the most convincing evidence would be to show exactly how these channel impairments looked in a cell.
For that they asked Dick, who studies the protein CaV1.2, a channel that shepherds calcium into the cell. Calmodulin triggers this channel to close once enough calcium has entered. Overgaard asked Dick to look specifically at whether the mutation impaired the closure of CaV1.2. Dick had never heard of the Folbigg case, but to prevent her team from introducing any bias, she relabelled dishes of cells to hide their provenance. Sure enough, the Folbigg variant delayed the channel closure, letting extra calcium into the cell. “That’s what we know to be one of the signatures of a pathogenic calmodulin mutation,” Dick says.
But that wasn’t the only effect the variant had. Wayne Chen, who studies calcium channels at the University of Calgary in Canada, was asked to conduct similar experiments on a ryanodine receptor, a channel that controls the release of calcium into the cell from intracellular stores. As it does with CaV1.2, calmodulin binds to ryanodine receptors and triggers the channels to close. This prompts the heart muscle to relax. When Chen’s team expressed the G114R variant in human cells, that channel had trouble closing, too. The combined effect of the variant on both channels will increase calcium in the cell, says Dick, which increases the chance of arrhythmia. “If you asked me, ‘Would this mutation be likely to cause sudden death?’, I would say somebody with this mutation is at very high risk of that,” she says...
For that they asked Dick, who studies the protein CaV1.2, a channel that shepherds calcium into the cell. Calmodulin triggers this channel to close once enough calcium has entered. Overgaard asked Dick to look specifically at whether the mutation impaired the closure of CaV1.2. Dick had never heard of the Folbigg case, but to prevent her team from introducing any bias, she relabelled dishes of cells to hide their provenance. Sure enough, the Folbigg variant delayed the channel closure, letting extra calcium into the cell. “That’s what we know to be one of the signatures of a pathogenic calmodulin mutation,” Dick says.
But that wasn’t the only effect the variant had. Wayne Chen, who studies calcium channels at the University of Calgary in Canada, was asked to conduct similar experiments on a ryanodine receptor, a channel that controls the release of calcium into the cell from intracellular stores. As it does with CaV1.2, calmodulin binds to ryanodine receptors and triggers the channels to close. This prompts the heart muscle to relax. When Chen’s team expressed the G114R variant in human cells, that channel had trouble closing, too. The combined effect of the variant on both channels will increase calcium in the cell, says Dick, which increases the chance of arrhythmia. “If you asked me, ‘Would this mutation be likely to cause sudden death?’, I would say somebody with this mutation is at very high risk of that,” she says...
For now Ms. Folbigg remains in prison, based on the circumstantial evidence that all four of her children dying couldn't be a coincidence.
If the cause of the children's death turns out to be genetic, a great injustice will have been done.
9 replies
 = new reply since forum marked as read
Highlight:
NoneDon't highlight anything
5 newestHighlight 5 most recent replies
= new reply since forum marked as read
Highlight:
NoneDon't highlight anything
5 newestHighlight 5 most recent replies
Did a genetic mutation lead to a woman being convicted of murdering her four children? [View all]
NNadir
Nov 2022
OP
I'm not an expert on this stuff mind you but two things jump quickly to mind
Hugh_Lebowski
Nov 2022
#1
Given the totality of what you explained, I'd be quite a bit more amenable to the idea that it was
Hugh_Lebowski
Nov 2022
#4
The jury, based on tainted (or a lack of) evidence seems to have agreed with you. Were I on...
NNadir
Nov 2022
#5
Thanks NNadir. I appreciate what a thoughtful, logical, and well-educated person you are.
Hugh_Lebowski
Nov 2022
#6
Thank you for your kind words. It was indeed a good week, and hopefully next week will be...
NNadir
Nov 2022
#7
Huntington's is dominant and children have a 50% chance of getting it from the affected parent.
NH Ethylene
Nov 2022
#8
I'm surprised she was convicted with no direct evidence that indicated murder.
NH Ethylene
Nov 2022
#9