The dependence of CO2 hydrogenation to methanol on the surface coordination of copper catalysts. [View all]
Last edited Sun Aug 27, 2023, 12:07 PM - Edit history (1)
The paper I'll briefly discuss in this post is this one: Coordination Environment Dependent Surface Cu State for CO2 Hydrogenation to Methanol Mengyang Song, Tangkang Liu, Xinlin Hong, and Guoliang Liu ACS Sustainable Chemistry & Engineering 2023 11 (32), 12135-12144
Hydrogen is a dirty fuel despite all of the delusional crap handed out about "green hydrogen." "Green hydrogen" is a Potemkin marketing tool to promote the use of dangerous fossil fuels.
A Giant Climate Lie: When they're selling hydrogen, what they're really selling is fossil fuels.
It's actually not really a "fuel" at all; it is primarily a synthetic intermediate necessary to maintain the world's food supply via the synthesis of ammonia based fertilizers. The energy value of the hydrogen were it a fuel, is about 1/2 the energy content of the dangerous fossil fuels used to make it.
(We have over in the wilderness industrialization forum at DU, fossil fuel salespeople and salesbots, rebranding fossil fuels as "hydrogen;" it's rather like the marketing of cigarettes in the 1940's and 1950's as health aids.)
However, in theory, although not in a practical sense, hydrogen could be used to make clean fuels, specifically methanol - which currently consumes about 10% of the world's hydrogen to manufacture, albeit as a solvent, not a fuel - or even better (far better actually) the dehydration product of methanol, dimethyl ether.
The use of hydrogen directly as a fuel, as is often advertised by fossil fuel salespeople and salesbots here and elsewhere, is border line insane, given the chemical, physical, and materials science issues connected with doing so.
As I often point out, there is a huge difference between the words could and is; the failure to make this distinction has left the planet in flames.
The only instance in which hydrogen can be a clean fuel is the case where it is made by exergy capture via process intensification using nuclear energy as the primary energy source. This could be developed but it isn't available now, and thus hydrogen remains what it has always been, an energy consuming captive intermediate.
Anyway, it would be nice to use copper catalysts efficiently as they are cheaper and more sustainable than other hydrogenation catalysts. I very much like the introduction to this paper despite the delusional reference to so called (and unsustainable) "renewable energy," because it references the 1994 by the late Nobel Laureate George Olah proposing a circular CO2 economy. This paper has hung in my mind for the last 30 years. The introduction of the paper currently under discussion:
Heterogeneous catalysts for methanol synthesis reactions mainly include Cu-based, noble metal-based (e.g., Au, Ag, Pd, and Pt), and oxide-based catalysts. (8−13) Among them, Cu-based catalysts hold great potential for practical application due to the advantages of low cost and ideal activity under relatively mild conditions. It is generally believed that the reactivity of Cu catalysts is strongly associated with the use of supports such as ZnO, (14,15) ZrO2, (16−18) Al2O3, (19) and CeO2. (20,21) The metal oxide supports can regulate the chemical environment of active Cu and the Cu-metal oxide interfaces usually act as active sites for methanol synthesis from CO2 hydrogenation. Metal–organic frameworks (MOFs) are a new class of porous materials created by the hybrid self-assembly of metal ions, metal clusters, and organic ligands, which have become one of the most striking materials in the field of catalysis, due to their advantages of adjustable pore size, large specific surface area, and high porosity. (22−27) MOF materials can anchor and confine metal ions/clusters in channels, cages, or defects, and the coordination microenvironment can tailor the chemical state of active metal, thus generating catalytic reactivity. (28) Besides, the porous structure of MOFs enables the formation of abundant metal–support interfaces, which provides an opportunity to investigate the connection between metal and support in the CO2 hydrogenation to methanol. (29,30) However, there are still some problems to be solved, such as the relationship between the coordination environment of MOFs and the chemical state of confined metal (e.g., Cu) and how to construct an active interface for efficient methanol synthesis.
In this work, we report that the chemical environment of Cu can be precisely tailored by changing the terephthalic acid linkers in the supporting Zr-MOFs (UiO-66 and UiO-66-NH2). It is found that the incorporated Cu2+ ions are coordinated with the carboxyl groups in UiO-66, while they are coordinated with the amino groups in UiO-66-NH2. Upon in situ hydrogen reduction, the Cu@UiO-66 catalyst possesses a high content of surface Cu+ species related to Cu-ZrO2 interfaces when compared to Cu@UiO-66-NH2. For the CO2 hydrogenation reaction, Cu@UiO-66 shows a high methanol production rate of 2.86 g gCu–1 h–1 at 1 MPa and 260 °C, which is 1.7 times that of Cu@UiO-66-NH2 and 6.0 times that of the commercial Cu/ZnO/Al2O3 catalyst. Such good activity can be attributed to the abundant Cu+-ZrO2 interfaces in the Cu@UiO-66 catalyst...
The authors discuss their approach to using Zr MOF's in some detail.
A graphic from the paper:
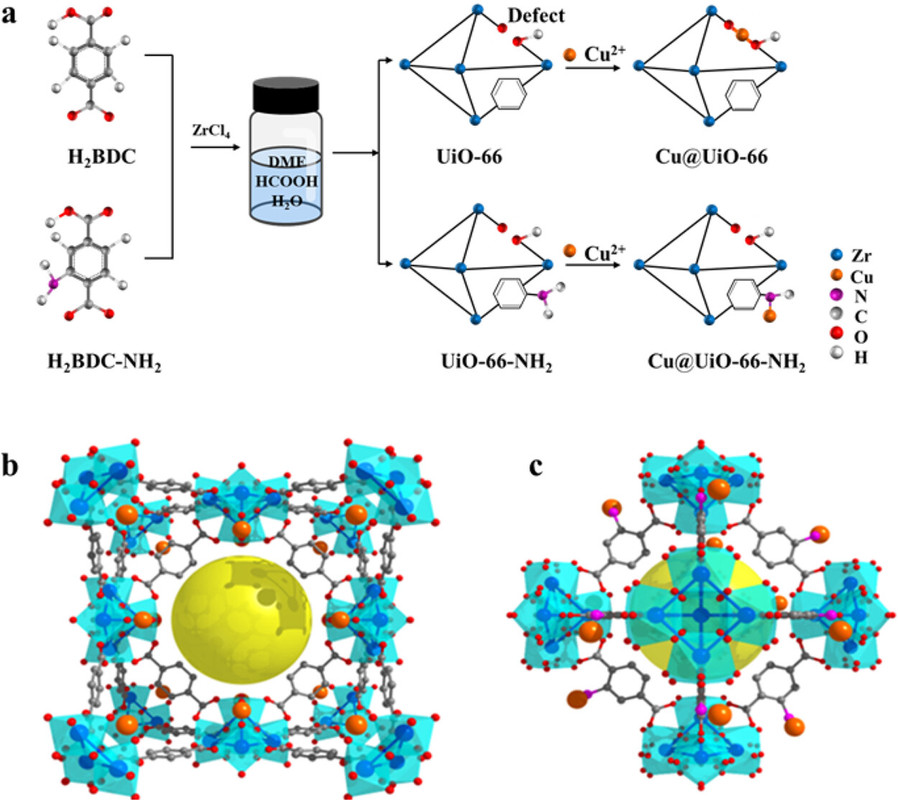
The caption:
The diagram because of the way the surface of the liquid line is labeled makes it appear as if the solvent for the MOF synthesis is DME; actually it is DMF and formic acid. Like DME, however, DMF and formic acid can both be produced by the hydrogenation of CO2, in the case of DMF, also requiring the hydrogenation of nitrogen gas, the Haber-Bosch process on which the world's food supply depends.
I trust you're having a pleasant weekend.